Description
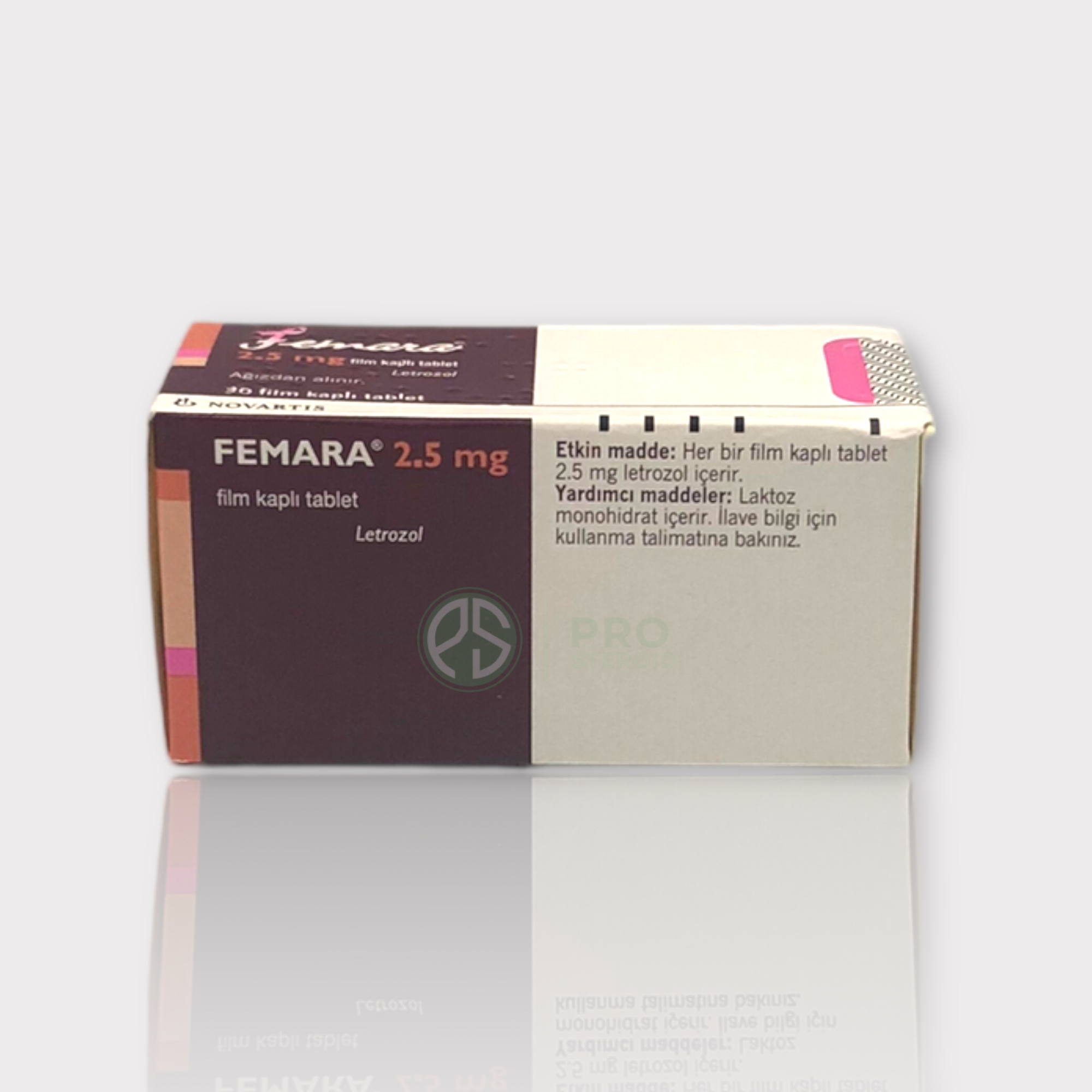
Femara (Letrozole 2,5 mg. | 30 tabs.)
Indications and usage
Adjuvant Treatment of Early Breast Cancer: Femara (letrozole) is indicated for the adjuvant treatment of postmenopausal women with hormone receptor-positive early breast cancer.
Extended Adjuvant Treatment of Early Breast Cancer: Femara is indicated for the extended adjuvant treatment of early breast cancer in postmenopausal women, who have received 5 years of adjuvant tamoxifen therapy. The effectiveness of Femara in the extended adjuvant treatment of early breast cancer is based on an analysis of disease-free survival (DFS) in patients treated with Femara for a median of 60 months.
First and Second-Line Treatment of Advanced Breast Cancer: Femara is indicated for first-line treatment of postmenopausal women with hormone receptor-positive or unknown, locally advanced or metastatic breast cancer. Femara is also indicated for the treatment of advanced breast cancer in postmenopausal women with disease progression following antiestrogen therapy.
Dosage and administration
Recommended Dose: The recommended dose of Femara is one 2.5 mg tablet administered once a day, without regard to meals.
Use in Adjuvant Treatment of Early Breast Cancer: In the adjuvant setting, the optimal duration of treatment with letrozole is unknown. In both the adjuvant study and the post approval adjuvant study, median treatment duration was 5 years. Treatment should be discontinued at relapse.
Use in Extended Adjuvant Treatment of Early Breast Cancer: In the extended adjuvant setting, the optimal treatment duration with Femara is not known. The planned duration of treatment in the study was 5 years. In the final updated analysis, conducted at a median follow-up of 62 months, the median treatment duration for Femara was 60 months. Seventy-one percent (71%) of patients were treated for at least 3 years and 58% of patients completed at least 4.5 years of extended adjuvant treatment. The treatment should be discontinued at tumor relapse.
Use in First and Second-Line Treatment of Advanced Breast Cancer: In patients with advanced disease, treatment with Femara should continue until tumor progression is evident.
Use in Hepatic Impairment: No dosage adjustment is recommended for patients with mild to moderate hepatic impairment, although Femara blood concentrations were modestly increased in subjects with moderate hepatic impairment due to cirrhosis. The dose of Femara in patients with cirrhosis and severe hepatic dysfunction should be reduced by 50%. The recommended dose of Femara for such patients is 2.5 mg administered every other day. The effect of hepatic impairment on Femara exposure in noncirrhotic cancer patients with elevated bilirubin levels has not been determined.
Use in Renal Impairment: No dosage adjustment is required for patients with renal impairment if creatinine clearance is greater than or equal to 10 mL/min.
Dosage forms and strengths: 2.5 mg tablets: dark yellow, film-coated, round, slightly biconvex, with beveled edges (imprinted with the letters FV on one side and CG on the other side).
Reviews
There are no reviews yet.